Fosamax does more harm than goodEvelyn PringleApr. 18, 2006 |
Popular 
FL State Sen. Randy Fine Celebrated Israel Killing an American - Trump Just Endorsed Him For Congress
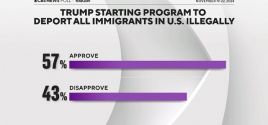
Poll: 57% of Americans Support Trump Starting Program to Deport All Illegal Immigrants

Russia Advancing in Ukraine at 'Fastest Pace Since Early 2022'

Trump Nominates Pam Bondi for Attorney General

Netanyahu Cries 'Antisemitism' After International Criminal Court Issues Warrant for His Arrest
 The osteoporosis drug Fosamax has been on the market for a little over 10 years now. Drug maker Merck promoted it heavily by selling women the fear of a disabling hip fracture and the necessity of regular bone-density tests. Merck's initial TV advertising campaign featured a slim woman in her mid-40s, conveying the notion that testing was appropriate for women in this age group. Fosamax belongs to a drug class known as bisphosphonates. Novartis's Aredia and Zometa injections are the two intravenous versions used in chemotherapy, and Merck's Fosamax and Procter and Gamble’s Actonel are the most commonly used oral versions of the drugs. Bisphosphonate in tablet form is commonly marketed to prevent and treat osteoporosis in post-menopausal women. Stronger forms are used to manage advanced cancers that have metastasized to the bone. For cancer therapy, the drugs are given intravenously, and usually for long periods of time. For many years, estrogen therapy was used to prevent osteoporosis, until 2002, when a study by the Women's Health Initiative said that estrogen posed more risks than benefits. When the study revealed that hormone therapy carried slight, but measurable, heart and breast cancer risks, prescriptions for bisphosphonates shot up 32 percent, according to IMS Health, which monitors pharmaceutical trends. However, although Fosamax may improve bone density, experts say when it comes to fracture prevention, its benefit is modest at best. In fact, some researchers say that when taken for more than 10 years, Fosamax will actually make bones more brittle and thus, more susceptible to fracture. And even if patients stop taking the drug, doctors say it can stay in the body for up to 10 years. In a 2004 letter published in the Annals of Internal Medicine, researcher Susan Ott, MD, of the University of Washington wrote: "Many people believe that these drugs are 'bone builders,' but the evidence shows they are actually bone hardeners." In a December 13, 2004, press release, doctors at Long Island Jewish (LIJ) Medical Center announced that they had discovered a link between a common chemotherapy drug and a serious bone disease called osteonecrosis of the jaw (ONJ). The discovery, published in the Journal of Oral and Maxillofacial Surgeons, prompted both the FDA and Novartis to issue warnings to physicians and dentists about the risk for the potential adverse effect. According to LIJ, “ONJ is a condition in which the bone tissue in the jaw fails to heal after minor trauma such as a tooth extraction, causing the bone to be exposed.” The exposure, the doctors said, can eventually lead to infection and fracture and may require long-term antibiotic therapy or surgery to remove the dying bone tissue. The chief of the Division of Oral and Maxillofacial Surgery at LIJ, Salvatore Ruggiero, DMD, MD, said they conducted the study after they noticed a cluster of cancer patients with necrotic lesions in the jaw, a condition they previously saw in only one or two patients a year. In conducting a review of the patients’ charts, the doctors found that the 63 patients, diagnosed with ONJ over a three-year period, shared one commonality, they all had received long-term bisphosphonate therapy. Of the 63 patients diagnosed between February 2001 and November 2003, fifty-six were cancer patients who had received infusions of bisphosphonates for at least a year, and seven other patients had been receiving long-term oral therapy for osteoporosis. “The patients developed ONJ after normal bone trauma,” the press release said, “such as a tooth extraction, while receiving bisphosphonate therapy.” Rather than healing, the bone began to die, and a majority of the patients required surgery to remove the diseased bone. Another study quoted on April 4, 2006, by United Press International, found more than 2,400 patients who were taking the injected form of bisphosphonate had suffered bone damage to their jaws since 2001. In addition to the 2,400 patients who were taking the injected form, the study found 120 patients taking the oral form of the drug who had been stricken with such incapacitating bone, joint, or muscle pain that some became bedridden and others required walkers, crutches or wheelchairs. While the number may seem small when compared to the estimated 39 million oral prescriptions written in 2005, health experts told The Los Angeles Times that the problems may show a trend. "We've uncovered about 1,000 patients (with jaw necrosis) in the past six to nine months alone, so the magnitude of the problem is just starting to be recognized," Kenneth Hargreaves, of the University of Texas, told the newspaper. "We're not quite sure what we're dealing with over the long haul,” Dr Susan Ott, told the Times. “Side effects like this should make ordinary, healthy women think twice," she warned. Christopher Loder, a spokesman for Merck, claims that ONJ with Fosamax is "exceedingly rare." But while this may appear to be true, experts say it is always good to consider that at least 90 percent of drug side effects go unreported to the FDA, so the actual number of people stricken with ONJ is likely to be much higher. In a statement, Merck said that in “all of our controlled clinical trials with Fosamax, which involved more than 17,000 patients, including some that were 10 years in duration, we had no reports," according to the April 3 LA Times. However, a closer look at the results of Merck studies casts a shadow on their favorable outcomes. For instance, Merck virtually controlled everything about a 2002 Annals of Internal Medicine paper praising the use of Fosamax. The paper’s lead author was Susan Greenspan, a Harvard Medical School professor and director of the Beth Israel Deaconess Osteoporosis Prevention and Treatment Center at the time. As it turns out, Merck paid for the recruitment and participation of all 327 clinical trial subjects; the company collected the data from 25 separate facilities; Merck employees handled “coordinating the early phases of the study” and provided “expertise in study conduct,” and in the end, Merck retained control and ownership of the research itself. Admittedly, most of these details are revealed in the Annals article’s disclosures and acknowledgments, but such qualifications rarely appear in articles on web sites, where many people learn about trial results. The year before Dr. Greenspan's paper was published in 2001, Fosamax sales barely reached $1 billion. The following year the drug had sales of $2.7 billion. On April 10, a lawsuit was filed against Merck, in a US District Court in Fort Meyers, Florida, alleging Fosamax is a defective product because it can cause osteonecrosis of the jaw, and also alleging that Merck concealed the drug’s dangerous side effects from doctors and patients. The lawsuit alleges that the FDA asked Merck to add a ONJ warning to Fosamax's label in August of 2004 and that it has yet to comply with that request. Merck claims it received a request from the FDA to update the label in January 2005, and says the warning was added in July 2005. However, the "label" referred to is actually a 22-page document that is provided to pharmacies that fill prescriptions for Fosamax, and the warning does not appear until page 13. The attorney in the Florida lawsuit is seeking class action status and the suit reportedly may represent more than 10 million Fosamax users. Fosamax is Merck's second best-selling drug with revenues of $3.2 billion in 2005, according to an April 12 Associated Press press. But analysts note that Merck is already bogged down with thousands of Vioxx lawsuits and many estimate the total bill for Vioxx related suits may reach between $20 to $30 billion. However, analyst Robert Hazlett of Suntrust Robinson Humphrey says that Merck has the resources to pay damages in the tens of billions of dollars. The company, he told CNN Moneyline, had $16.7 billion in cash and investments on its balance sheet as of the end of December 2005. Information for injured parties can be found at Lawyers and Settlements. Evelyn Pringle is a columnist for Independent Media TV and an investigative journalist focusing on exposing corruption in government. |



