With Tysabri decision, the FDA declares no drug is too dangerous to be FDA approvedNewsTargetMar. 28, 2006 |
Popular 
FL State Sen. Randy Fine Celebrated Israel Killing an American - Trump Just Endorsed Him For Congress
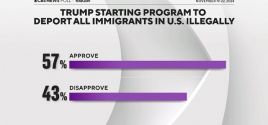
Poll: 57% of Americans Support Trump Starting Program to Deport All Illegal Immigrants

Russia Advancing in Ukraine at 'Fastest Pace Since Early 2022'

Trump Nominates Pam Bondi for Attorney General

Netanyahu Cries 'Antisemitism' After International Criminal Court Issues Warrant for His Arrest
 The U.S. Food and Drug Administration, the agency that claims to be responsible for protecting consumers from dangerous food and drug products, has just surrendered its primary responsibility. Recently, an FDA advisory panel voted to recommend that a dangerous prescription drug Tysabri, which was withdrawn from the market a year ago due to its promoting of a deadly brain disease, should now be put back on the market. But here's the really shocking part: The justification for this decision to reinstate a drug with known deadly side effects is based on the idea that patients should now weigh the risks of dangerous drugs and decide for themselves whether the risks outweigh the benefits, if any. Stop the music for a minute. Do you realize that with this decision, the FDA has just rendered itself irrelevant? If patients are going to be held responsible for making risk vs. benefits decisions on prescription drugs, then why do we need the FDA at all? As you may have guessed, there are enormous problems with this new stance by the FDA. The first is that patients do not have the medical knowledge to understand and interpret the significance of these side effects that will no doubt only be mentioned in small print somewhere on a piece of paper that most patients will probably ignore. How can the FDA justifiably turn over safety decisions on deadly drugs to patients? The second problem with this new stance by the FDA is that it exposes a wicked double standard: With prescription drugs, patients should be able to weight benefits vs. risks, even for drugs that may kill you. But with herbs and nutritional supplements, no such decision is extended to patients. The FDA merely bans whatever natural substances it wishes, usually based on reports of very small numbers of people being harmed be extremely rare overdoses (such as with ephedra). In those cases, the FDA proudly proclaims it is, "Protecting everyone from a dangerous herb!" Related book: Dispensing with the Truth : The Victims, the Drug Companies, and the Dramatic Story Behind the Battle over Fen-Phen Mary Linnen, 29, was determined to lose 25 pounds before her wedding. In May 1996, her doctor prescribed a combination of drugs known as Fen-Phen. When Linnen complained of dizziness and shortness of breath... continues... (Concept: the FDA) In other words, the FDA now sees its job as protecting the public from "dangerous" herbs while shirking safety responsibilities on truly dangerous prescription drugs. It's up to the public to decide whether deadly drugs are worth the risk, according to the FDA. But when one herb which has been safely used for 5,000 years in Chinese medicine happens to harm 20 people who overdosed in a mad weight loss frenzy, the FDA bans it "to protect everyone!" The FDA's position now comes down to simply this: Everyone needs to be protected from herbs and nutritional supplements, but no one needs to be protected from prescription drugs. And this now completes the full reversal of the FDA. The agency now has both feet squarely in Bizarro world. In doing this, I wonder if the FDA realizes it has made itself irrelevant. If the agency is now merely going to pass through drug safety decisions to doctors and patients, then why do we need the FDA at all? The agency is no longer a gatekeeper. It is a toll booth, where drug companies pay a toll on their way to customers. And apparently, the toll fee is happily accepted regardless of whether the drug in question helps people or kills them. I've often said that no drug is too dangerous to meet FDA safety requirements, and now the FDA has proven it. Even a drug that outright kills patients with a painful, horrifying death will now be FDA approved. There is no longer even the concept of safety standards at the FDA. Now, there is merely avoidance of assessing safety. The agency that was once tasked with actually regulating the drug industry has now become its largest marketing department. The concept of drug "safety" is now history. Any drug, no matter how dangerous, is now qualified for FDA approval. If thalidomide were a new drug today, the FDA would no doubt happily approve it for any use as long as it carried a small-print warning about its side effects. In fact, we may soon see the FDA (arm-in-arm with Big Pharma marketing reps) bringing back all sorts of deadly, dangerous drugs over the next few years. Drugs that were once withdrawn from the market due to outrageous side effects (such as Vioxx, which reportedly caused the death of tens of thousands of Americans) will now be re-approved and dumped onto patients who must now make their own safety assessments of patented, synthetic chemicals. |



