Record Sales of Sleeping Pills Are Causing WorriesNY TimesFeb. 13, 2006 |
Popular 
FL State Sen. Randy Fine Celebrated Israel Killing an American - Trump Just Endorsed Him For Congress
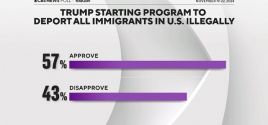
Poll: 57% of Americans Support Trump Starting Program to Deport All Illegal Immigrants

Russia Advancing in Ukraine at 'Fastest Pace Since Early 2022'

Trump Nominates Pam Bondi for Attorney General

Netanyahu Cries 'Antisemitism' After International Criminal Court Issues Warrant for His Arrest
 Americans are taking sleeping pills like never before, fueled by frenetic workdays that do not go gently into a great night's sleep, and lulled by a surge of consumer advertising that promises safe slumber with minimal side effects. About 42 million sleeping pill prescriptions were filled last year, according to the research company IMS Health, up nearly 60 percent since 2000. But some experts worry that the drugs are being oversubscribed without enough regard to known, if rare, side effects or the implications of long-term use. And they fear doctors may be ignoring other conditions, like depression, that might be the cause of sleeplessness. Although the newer drugs are not believed to carry the same risk of dependence as older ones like barbiturates, some researchers have reported what is called the "next day" effect, a continued sleepiness hours after awakening from a drug-induced slumber. Ten percent of Americans report that they regularly struggle to fall asleep or to stay asleep throughout the night. And more and more are turning to a new generation of sleep aids like Ambien, the best seller, and its competitor, Lunesta. Experts acknowledge that insomnia has become a cultural benchmark — a side effect of an overworked, overwrought society. "Clearly, there's a significant increase in people who report insomnia and, from my perspective, that is the result of our modern-day lifestyle," said Dr. Gregg D. Jacobs, a psychologist and assistant professor of psychiatry at Harvard. Or at least that is an impression that drug makers are clearly trying to capitalize on, he said. And that concerns him and some other researchers who warn that despite their advertised safety, the new generation of sleep aids can sometimes cause strange side effects. The reported problems include sleepwalking and short-term amnesia. Steven Wells, a lawyer in Buffalo, said he started using Ambien last year because his racing mind kept him awake at night. But he quit after only one month, concerned about several episodes in which he woke up to find he had messily raided the refrigerator and, finally, an incident in which he tore a towel rack out of a wall. "The weird thing was that I had no recollection of it the next day," said Mr. Wells, who added that he found the episodes frightening. Ambien's maker, Sanofi-Aventis, said the drug had been used for 12 billion nights of patient therapy. "When Ambien is taken as prescribed, it's a safe and effective treatment," said Emmy Tsui, a company spokeswoman. A Food and Drug Administration spokeswoman, Susan Cruzan, said she was not aware of an unusual number of complaints with the drugs. Drug makers spent $298 million in the first 11 months of 2005 to convince consumers that the sleep aids are safe and effective. That was more than four times such ad spending in all of 2004. In the last year, much of the advertising surge has been a result of competition from Lunesta, which the drug maker Sepracor introduced last April to compete with Ambien. Through November, Sepracor led the sleeping pill advertising field, spending more than $185 million, according to figures from TNS Media Intelligence, which did not have final figures for December. In response, Sanofi-Aventis, marketing both Ambien and its controlled-release version, Ambien CR, spent $107 million from last January through November, according to TNS. That was nearly double its ad spending on Ambien in 2004. Even the most infrequent television viewers would have trouble missing the Lunesta ads, which feature a luna moth fluttering around the bed of a peaceful sleeper. Dr. Jacobs said that in one hour of prime-time television recently, he saw three ads for sleeping pills: two for Lunesta and another for Ambien. "You've got the patient population being bombarded with advertising on TV," Dr. Jacobs said. "You've got increased advertising to physicians. You've got a formula for sales going up dramatically." One financial analyst, Jon LeCroy of Natexis Bleichroeder, said Lunesta's ad campaign last fall was tied to the new season of "Desperate Housewives," whose audience is about 55 percent female. Studies have shown that women have insomnia more frequently than men. Last week, Sepracor's stock jumped $8.53 in one day, after Sepracor reported a profit and remarkably strong use of Lunesta in its first year on the market, with sales of $329 million. More than 213,000 doctors wrote 3.3 million prescriptions for it last year, the company says. Sepracor announced the addition of 450 people to its current sales force of 1,500 to increase marketing of the drug to physicians. Sanofi-Aventis, with a sales force of 3,000, is working to shift patients from Ambien, which loses its patent protection in October, to the newer version, Ambien CR. The newer pill has a quickly dissolving outer layer meant to immediately induce sleep, with a slower-dissolving inner layer to sustain sleep. Another drug in the class is Sonata, marketed by King Pharmaceuticals. Because it is short acting, Sonata is recommended for people who have trouble falling asleep but no trouble staying asleep. Drugs in the class are frequently referred to as "Z" drugs, a play on both their effect and the Z's in their generic names, like zolpidem (Ambien) and eszopiclone (Lunesta). All aim at a brain neurotransmitter that is believed to reduce neural activity. Another new entrant to the market, Rozerem, by the Japanese company Takeda Pharmaceuticals, has been available in drugstores since September but has not yet been heavily advertised. The drug works by a different mechanism from the others, acting on the brain's melatonin receptors, which are believed to play a role in sleep-wake cycles. Mr. LeCroy, the analyst, who is also a medical doctor, predicts the advertising will intensify if Neurocrine Biosciences and its partner Pfizer are permitted to introduce their new sleeping pill, Indiplon; an F.D.A. decision on that is expected in May. "That's going to make the competition get more cutthroat," Mr. LeCroy said, predicting that the market for branded sleeping pills, currently about $2 billion a year, could grow to $3.8 billion, even with Ambien set to go generic. "This is only going to get crazier." The Carlat Psychiatry Report, a newsletter by Dr. Daniel J. Carlat, a psychiatrist in Newburyport, Mass., reviewed the Z drugs recently and concluded that their differences were merely subtle. But Dr. Carlat warned that Lunesta, because it was longer acting, was more likely to cause next-day sleepiness problems "in comparison with some of its cousins." Dr. Carlat cited a 1998 study in Britain, published in The Lancet, which found that taking zopiclone, the compound known as the "mother" of Lunesta and marketed in Europe, was linked to an increased risk of automobile accidents. But Sepracor's chief financial officer, David P. Southwell, said that Lunesta, while a chemical variant of zopiclone, was a totally different drug. He referred a reporter to the F.D.A.-approved label, which lists clinical studies of next-day effects showing there was no consistent pattern of impaired mental functioning the day after Lunesta use. The possible role of Ambien was investigated in connection with well-chronicled transportation disasters in 2003 — the crash of the Staten Island Ferry, which killed 11 passengers, and an accident involving a Texas church bus in Tallulah, La., which killed 8 passengers. The assistant captain who was piloting the ferry, like the bus driver, had a prescription for Ambien, but there was no evidence either had taken it before the crashes. Dr. David G. Fassler, a clinical professor of psychiatry at the University of Vermont College of Medicine, said he was concerned that the heavy marketing and prescribing of the sleep medications would lead to use in patients who have underlying conditions that are left untreated. "I'm concerned that difficulty sleeping can be a sign of multiple disorders, including problems with anxiety and depression," he said, expressing worry that patients who are not thoroughly evaluated might be treated for their insomnia while other problems, like anxiety or decreased appetite, are not addressed. In clinical trials, the most common side effect of the drugs, however, is that people wake up feeling sleepy the next day. Dr. Daniel J. Buysse, a University of Pittsburgh psychiatrist who has consulted for the industry on sleeping pills, said they were a rare example of drugs in which the desired effect and the major side effect were the same thing. "One occurs when you want it, and the other occurs when you don't," he said. Another problem associated with using sleeping pills is a condition commonly called traveler's amnesia, in reference to the frequent use by people who travel across time zones. Such amnesia can occur when people return to daytime activities too quickly after taking the drugs. The labels carry warnings that the drugs should be used only when people can devote a full night to sleeping. In some cases, however, users have reported that they awakened during the middle of the night in sleepwalking states, but — like Mr. Wells, the lawyer in Buffalo — had no recollection of their activities. The amnesiac effects of Ambien were a factor in the acquittal last week of a United States Air Force linguist who had been charged with raping a colleague while the two were stationed in Qatar. The woman who said she was the victim, also a linguist, testified that she was not sure whether the incident was a dream because she had taken Ambien, according to the Stars and Stripes report on the military trial, which occurred in Britain. Dr. Buysse said such bouts of nocturnal uncertainty occur occasionally with various Z drugs. "There have been some case reports of people who have been sleepwalking only when taking the drug," Dr. Buysse said. "I think it's rare, and it's the kind of thing that no one is going to have a very good estimate of. But if it happens to you, who cares if you're the only person of many?" |



