Johnson & Johnson to Run COVID-19 Vaccine Trials on InfantsChris MenahanInformationLiberation Mar. 08, 2021 |
Popular 
Schumer Moves to Silence Criticism of Israel as Hate Speech With 'Antisemitism Awareness Act'

As Poll Finds Ukrainians Want to End War, U.S. Pushes Zelensky to Bomb Russia and Expand Conscription

FBI Pays Visit to Pro-Palestine Journalist Alison Weir's Home

Netanyahu Cries 'Antisemitism' After International Criminal Court Issues Warrant for His Arrest

Federal Judge Orders Hearing Into Questionable 'Auction' of Infowars to The Onion
  Johnson & Johnson is planning to conduct COVID-19 vaccine trials on infants and newborns -- even though the CDC's own data shows they face virtually zero risk of death from the virus. Johnson & Johnson is planning to conduct COVID-19 vaccine trials on infants and newborns -- even though the CDC's own data shows they face virtually zero risk of death from the virus.The CDC's data shows young people aged 0-19 have a 99.997% survival rate. 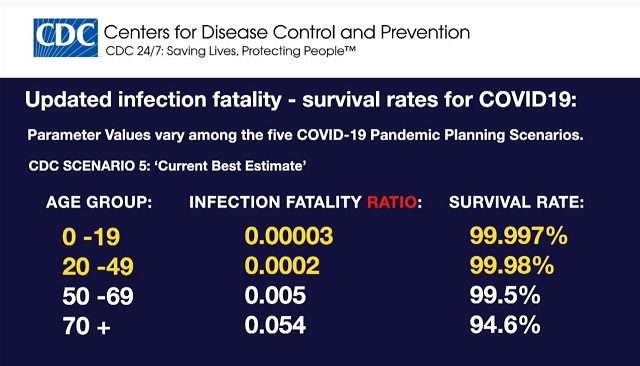 From The New York Times, "Johnson & Johnson has planned trials of its vaccine that will include infants": Johnson & Johnson plans to test its coronavirus vaccine in infants and even in newborns, as well as in pregnant women and in people who have compromised immune systems.  When Dr. Levy saw the outlines of the planned trials, "they turned my head," he said. They were reported as part of the company's application to the F.D.A. for emergency use approval and discussed at the F.D.A. meeting.Children's Health Defense has more on the vaccine: Rather than use the messenger RNA (mRNA) technology being deployed for the first time in the Pfizer and Moderna injections, J&J's vaccine (made by the company's Janssen Pharmaceuticals subsidiary) features a genetically engineered "viral vector" design reliant on a weakened common-cold virus called adenovirus 26.This is elective medical experimentation on infants and newborns which it appears is being approved under an "emergency use authorization" for a non-emergency. Johnson & Johnson's own data shows their vaccine is at best 72% effective in America and only 57% effective in South Africa, where a different strain is spreading. "At this time, data are not available to determine how long the vaccine will provide protection, nor is there evidence that the vaccine prevents transmission of SARS-CoV-2 from person to person," the FDA said upon approving the vaccine under a EUA. KOCO 5 News reported last week that Moderna is conducting similar trials with their mRNA vaccine on children as young as 2. "The Lynn Institute is working with about 300 volunteers in the 12 to 16 age group for a Moderna vaccine trial," KOCO reported. "In a few months, they'll start with patients ages 2 to 12." [This article was updated on Monday due to uncertainty on whether the FDA has fully signed off on J&J's plan to extend the trials to infants and newborns as part of their emergency use authorization. My headline originally said the FDA had "approved" the planned J&J trials on infants. The planned infant trials were included in J&J's EUA application on page 34 and the NYT and others are reporting on the trials as though they're a certainty but I'm not 100 percent positive that the FDA signed off on the trials as part of the EUA. J&J's plans were clearly met with approval from the FDA's Dr. Ofer Levy and similar trials extending to children as young as 2 are moving forward with Moderna's mRNA vaccine but I'm not certain whether their plans to extend the trial to infants and newborns have been fully approved by the FDA. The situation should become clearer over the next few days or weeks.] [Header image via Beth on Flickr CC BY-SA 2.0 with headline from NY Mag's Intelligencer superimposed.] Follow InformationLiberation on Twitter, Facebook, Gab, Minds, Parler and Telegram. |



