Study: Cardiac Drug Doubles Risk of Kidney FailureConsumeraffairs.comJan. 30, 2006 |
Popular 
FL State Sen. Randy Fine Celebrated Israel Killing an American - Trump Just Endorsed Him For Congress
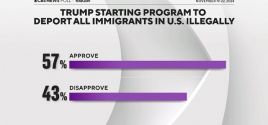
Poll: 57% of Americans Support Trump Starting Program to Deport All Illegal Immigrants

Russia Advancing in Ukraine at 'Fastest Pace Since Early 2022'

Trump Nominates Pam Bondi for Attorney General

Netanyahu Cries 'Antisemitism' After International Criminal Court Issues Warrant for His Arrest
 Aprotinin -- a drug approved by the FDA, marketed internationally for the last 13 years, and given to an estimated one million surgery patients to limit bleeding -- has now been proven to double a patient's risk of kidney failure, and increase the risk of heart attack, heart failure, and stroke. The results, published in this week's New England Journal of Medicine, are based on an independent, non-commercial, observational study conducted by The Ischemia Research and Education Foundation (IREF). "Our study provides compelling evidence of aprotinin's serious risks, and strongly suggests discontinuation of use and replacement with either of the two alternative generic and far less costly medications proven safe in this study," said IREF founder and principal scientist, Dennis T. Mangano, Ph.D., M.D. "Certainly, our findings -- coming on the heels of the Vioxx experience -- indicate that the problem of drug safety is not only ubiquitous, but also much more elusive than previously thought." In fact, Mangano said, the findings raise even more troubling concerns, for: (1) aprotinin has been on the market for three times as long as Vioxx, yet few comprehensive safety studies have been conducted since approval; (2) the life-threatening complications with aprotinin occurred far more frequently than those with Vioxx; and (3) far less expensive generic alternatives to aprotinin which are equally effective in limiting bleeding have been available, but have been underused. The article states that replacing aprotinin with one of two safe generic drugs would annually prevent as many as 11,050 dialysis complications, save at least $1 billion in healthcare (dialysis) costs, and reduce drug costs by at least $250 million. Each year approximately one million patients worldwide undergo surgical treatment following a heart attack, with the majority of these patients receiving one of three antifibrinolytic agents to limit blood loss during surgery: aprotinin (Bayer Healthcare Pharmaceuticals, Inc.), aminocaproic acid (generic), or tranexamic acid (generic). The two generic drugs have proven safe in limiting blood loss, and do not have the harmful effects of aprotinin. Patients scheduled for cardiac surgery should consult their physicians and avoid this risk, Mangano said. Aprotinin was approved by the U.S. Food and Drug Administration in 1993 and is manufactured by Bayer under the brand name Trasylol. Over the past three years, Trasylol sales have accelerated, with 2006 sales projected in excess of $600 million. "We estimate that as many as 10,000 patients may be unnecessarily on dialysis today due to aprotinin use. This serious impact on human lives underscores once again the necessity for meticulous, post -- approval surveillance, as well as ongoing, unbiased analysis of drug safety -- all conducted by entirely independent entities," said Mangano. "This is easier said than done, however, for the economic forces are -- and will continue to be -- substantial, with little corporate incentive to identify safety problems once drugs are approved and marketed." The New England Journal of Medicine article documents how aprotinin use was associated with a two-fold increase in renal failure requiring dialysis in patients undergoing both complex coronary artery surgery and primary surgery, excluding prior cardiac and current valve surgery. Among primary surgery patients, Dr. Mangano and colleagues found that aprotinin use also increased risk of myocardial infarction (48 percent), heart failure (109 percent), and stroke (181 percent). Neither of aprotinin's generic competitors, aminocaproic acid and tranexamic acid, was associated with increased renal, cardiac or cerebral events. Aprotinin is at least ten times more expensive than its generic competitors. |



