New York State Rescinds Mandatory H1N1 Vaccine for Healthcare Workersby Richard GaleProgressive Radio Network Oct. 26, 2009 |
Popular 
FL State Sen. Randy Fine Celebrated Israel Killing an American - Trump Just Endorsed Him For Congress
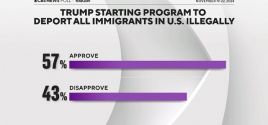
Poll: 57% of Americans Support Trump Starting Program to Deport All Illegal Immigrants

Trump Nominates Pam Bondi for Attorney General

Netanyahu Cries 'Antisemitism' After International Criminal Court Issues Warrant for His Arrest

Matt Gaetz Withdraws from Consideration as Attorney General
 People power is alive and well in New York State as evidenced by Governor David Patterson’s and his Health Commissioner’s decision on October 22 to immediately suspend the mandatory swine flu vaccine for the majority of New York’s healthcare workers. The official “imprint” is that the decision is based on the shortages in vaccine lots; however, vibrant demonstrations in Albany, massive letter writing campaigns, two separate lawsuits, and blistering testimonies by vaccination critics before the state’s health committee in New York City clearly played a major role in the decision. See Dr. Gary Null’s testimony to the New York State Health Oversight Legislative Committee While this is an important victory for health freedom, Peter Kauffmann, a spokesperson for Governor Patterson, told the Times Union that the government wants the supply to be available for “high risk groups,” which means small children, pregnant mothers, and the elderly. For this reason, the struggle continue because the H1N1 and seasonal influenza vaccines remain to be proven clinically safe and effective for these groups. Very recent and compelling independent research now shows that the swine flu threat may peak much earlier than being predicted by the WHO and the US health officials. The “high risk” groups, particularly children, will remain unprotected. The Purdue University study published on October 15 in the journal Euro Surveillance assessed the efficacy of the CDC’s vaccination program. The study concluded that swine flu infection “will peak so early that the planned CDC vaccination campaign will likely not have a large effect on the total number of people ultimately infected by the pandemic H1N1 influenza virus.” The study also found that the vaccination program will likely achieve only a 6 percent reduction in the number of infected people. More important, in children being targeted as “high risk,” vaccine immunity will not be achieved until “at least four weeks after vaccination and would occur too late in the pandemic to make a significant difference in the number of infected in that age group.”[1] Earlier, the Cochrane Database Collaboration’s meta-analysis of all available influenza studies conducted over several decades revealed that there is “no convincing evidence that [flu] vaccines can reduce mortality, [hospital] admissions, serious complications and community transmission of influenza. In young children below the age of 2, we could find no evidence that the vaccine was different from a placebo.”[2] And just yesterday, a CBS news exclusive reported on a three-month CBS investigation from state-to-state flu test results revealing that the government infection figures are erroneous and that those diagnosed with “probable” or “presumed” H1N1 infection have slim chances that they contracted the H1N1 virus.[3] In 2004, the nation’s Advisory Committee on Immunization Practices (ACIP) issued its recommendations on influenza vaccination that have remained today’s standard policy. A critical review of the ACIP’s document was published in the Journal of American Physicians and Surgeons, deconstructing the government health agencies’ arguments to promote the campaign for the vaccination of pregnant women. The study’s authors, Drs. Edward Yazbak and David Ayoub, unveil the very limited data the ACIP relied upon to make their decision that thimerosal in vaccines is safe. In addition, the government’s data to claim vaccine effectiveness was poor. The ACIP ignores the many clinical studies showing thimerosal’s high toxicity to fetal development in animal studies.[3] Dangers to vaccinated pregnant women and their developing babies remain. Every inactivated H1N1 vaccine package insert claims: no animal reproduction studies to observe potential fetal injury have been conducted for H1N1 vaccines. It is unknown whether the vaccine is excreted in human milk, and no studies have been completed to determine whether or not the vaccines and their ingredients are carcinogenic or impair fertility. In the face of very limited, poorly designed and executed clinical trials conducted by the vaccine makers, a separate lawsuit has been filed against the FDA by health freedom attorney Jim Turner and plaintiff Dr. Gary Null. That lawsuit is pending action and demands the FDA require independent safety trials on the H1N1 vaccine and independent oversight with full public disclosure. [1] Towers S, Feng Z. “Pandemic H1N1 influenza: predicting the course of a pandemic and assessing the efficacy of the planned vaccination program in the United States” Euro Surveillance. 2009; 14(41). [2] Reaney, Patricia. “No Evidence Flu Shots Work for Under-2s: Study. Reuters, September 22, 2005; Jefferson, Tom. “Safety of influenza vaccines in children.” The Lancet, 2005. 366:803-804. [3] CBS News: Swine Flu Cases Overestimated. www.cbsnews.com/stories/2009/10/21/cbsnews_investigates/main5404829.shtml [3] Ayoub D, Yazbak E. “Influenza vaccination during pregnancy: a critical assessment of the recommendations of the Advisory Committee on Immunization Practices.” Journal Amer Phys. and Surg. 11 (2): Summer 2006. Richard Gale is the Executive Producer of the Progressive Radio Network and a former Senior Research Analyst in the biotechnology and genomic industry. |



