Swine Flu Injections May Pose Contamination RiskNick Miller and Julia MedewThe Age Aug. 23, 2009 |
Popular 
FL State Sen. Randy Fine Celebrated Israel Killing an American - Trump Just Endorsed Him For Congress
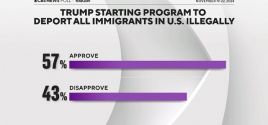
Poll: 57% of Americans Support Trump Starting Program to Deport All Illegal Immigrants

Trump Nominates Pam Bondi for Attorney General

Netanyahu Cries 'Antisemitism' After International Criminal Court Issues Warrant for His Arrest

Matt Gaetz Withdraws from Consideration as Attorney General
 LEADING infectious disease experts have called on the Federal Government to abandon its mass swine-flu vaccination plan because of fears the vaccine is a contamination risk that could spread blood-borne diseases. Health Minister Nicola Roxon yesterday announced that the Government would start deploying its first batch of swine-flu vaccine in coming weeks, with an aim to vaccinate as many people as possible to prevent further spread of the virus. But in a letter sent to Commonwealth Chief Medical Officer Jim Bishop, the Australasian Society for Infectious Diseases expresses deep concern about CSL's use of multi-dose vials for the vaccine and urged the Government to abandon its plan until it had single-dose vials. The letter, written by the Society's president, Associate Professor Tom Gottlieb, says multi-dose vials - bottles containing many doses of the vaccine - had been shown on many occasions to transmit infectious diseases, ''resulting in considerable morbidity and mortality''. To prevent contamination, clinicians must follow stringent infection-control guidelines and use new syringes and needles for every vaccination. ''Many members are concerned that there is a risk of adverse outcomes if a mass vaccination campaign was conducted using multi-dose vials,'' the letter says. It adds that it would be difficult to guarantee proper procedures were followed in hospitals, ''let alone clinics and general-practice units in the community, where there may be a lesser safeguarding of the necessary safe infection-control practices''. While the risk was slight, failures linked to the use of multi-dose vials could undermine confidence in other vaccination programs. Other mass vaccination programs in Australia - such as for infants, seasonal flu and cervical cancer - use single-dose vials. Professor Gottlieb said many bodies, including the World Health Organisation, recommend single-dose vials. Multi-dose vials in Australia had been linked to the transmission of HIV from a patient to four other people in a Sydney surgeon's rooms in 1989, he said. Overseas, they had been linked to outbreaks of hepatitis, HIV and bacterial diseases. Professor Gottlieb said that although his members - the bulk of infectious disease clinicians - supported vaccination, they wanted the Government to get this program right. ''A number of people are starting to wonder if we are rushing at a time when we're seeing that the peak has passed,'' he said. One month ago, the Royal Australian College of General Practitioners issued new draft guidelines for the use of multi-dose vials. Professor Jim Bishop said the Government's pandemic plan had always included the use of multi-dose vials because they could be rolled out quickly and were more efficient. ''The best evidence is that the epidemic in Australia has not yet clearly peaked, so ongoing protection of the vulnerable is needed,'' he said. ''General practices peak bodies are comfortable and believe the GP workforce is competent with the use of multi-dose vials.'' CSL said multi-dose vials had been chosen to help get the vaccine out more quickly. Spokeswoman Dr Rachel David said it would take ''many, many more months'' to produce single-dose vials and that another multi-dose vial flu vaccine it exports to the US had been used safely for the last three years. ''If multi-dose vials are used correctly there is no increased risk,'' she said. Ms Roxon said it would be the biggest vaccination program in the shortest period of time that Australia has undertaken. |



