Link between swine flu jab and deadly Guillain-Barré syndrome will be probed: Neurologists have been ordered to monitor whether new swine flu vaccinations could trigger a deadly nerve diseaseBy Laura Donnelly, Health CorrespondentThe Telegraph Aug. 17, 2009 |
Popular 
FL State Sen. Randy Fine Celebrated Israel Killing an American - Trump Just Endorsed Him For Congress
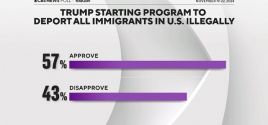
Poll: 57% of Americans Support Trump Starting Program to Deport All Illegal Immigrants

Trump Nominates Pam Bondi for Attorney General

Netanyahu Cries 'Antisemitism' After International Criminal Court Issues Warrant for His Arrest

Matt Gaetz Withdraws from Consideration as Attorney General
  Must see Old 60 Minutes Exposé On 1976 Swine Flu Propaganda, Debilitating Vaccines
Must see Old 60 Minutes Exposé On 1976 Swine Flu Propaganda, Debilitating VaccinesIts letter refers to the use of a swine flu vaccine in the United States in 1976, when 25 people died from GBS, while just one died from swine flu. The syndrome, which can be fatal, attacks the lining of the nerves, causing paralysis and inability to breathe. Concerns have already been raised that the new vaccine has not been sufficiently tested and that the effects, especially on children, are unknown. The jabs being developed by pharmaceutical companies and will be given to about 13 million people during the first wave of the programme, expected to start in October. Priority will be given to everyone aged six months to 65 with an underlying health problem, pregnant women and health professionals. In a letter sent by the HPA on July 29, neurologists have been asked to monitor closely any cases of GBS as the vaccine is rolled out. It alerts them to the use of a swine flu vaccine in the US in 1976, which was followed by more than 500 cases of GBS, including 25 deaths. The US programme was stopped after 10 weeks because of concerns over its safety, and the Government paid out millions of dollars in compensation to those affected. The swine flu virus in the new vaccine is a different strain from the 1976 virus, but the possibility of an increased incidence of GBS remains a concern. According to the Mail on Sunday, two letters were posted together to neurologists advising them of the concerns. The first, dated July 29, was written by Professor Elizabeth Miller, head of the HPA's Immunisation Department. It says: "The vaccines used to combat an expected swine influenza pandemic in 1976 were shown to be associated with GBS and were withdrawn from use. "GBS has been identified as a condition needing enhanced surveillance when the swine flu vaccines are rolled out. "Reporting every case of GBS irrespective of vaccination or disease history is essential for conducting robust epidemiological analyses capable of identifying whether there is an increased risk of GBS in defined time periods after vaccination, or after influenza itself, compared with the background risk." A second letter from the Association of British Neurologists is written by Dr Rustam Al-Shahi Salman, chairman of its surveillance unit (BNSU), and Professor Patrick Chinnery, chairman of its clinical research committee. It says: "Traditionally, the BNSU has monitored rare diseases for long periods of time. However, the swine influenza (H1N1) pandemic has overtaken us and we need every member's involvement with a new BNSU survey of Guillain-Barré syndrome that will start on August 1 and run for approximately nine months. "Following the 1976 programme of vaccination against swine influenza in the US, a retrospective study found a possible eight-fold increase in the incidence of GBS. Active prospective ascertainment of every case of GBS in the UK is required. Please tell BNSU about every case." Last night, the HPA insisted that it did not expect to find links between the new vaccine and GBS, and said it wanted to monitor any connection between the flu virus itself and the syndrome. Professor Miller at the HPA said: "This monitoring system activates pandemic plans that have been in place for a number of years. We'll be able to get information on whether a patient has had a prior influenza illness and will look at whether influenza itself is linked to GBS. "We are not expecting a link to the vaccine but a link to disease, which would make having the vaccine even more important." The UK's medicines watchdog, the Medicines and Healthcare Products Regulatory Agency, is already monitoring reported side effects from Tamiflu and Relenza and it is set to extend that surveillance to the vaccine. A Department of Health spokesman said: "The European Medicines Agency has strict processes in place for licensing pandemic vaccines. "In preparing for a pandemic, appropriate trials to assess safety and the immune responses have been carried out on vaccines very similar to the swine flu vaccine. The vaccines have been shown to have a good safety profile. "It is extremely irresponsible to suggest that the UK would use a vaccine without careful consideration of safety issues. The UK has one of the most successful immunisation programmes in the world." |



