Suicide and CymbaltaEli Lilly Markets Its Dangerous New Drug as If a Pipeline Depended On ItBy Martha Rosenberg Apr. 15, 2008 |
Popular 
FL State Sen. Randy Fine Celebrated Israel Killing an American - Trump Just Endorsed Him For Congress
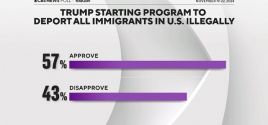
Poll: 57% of Americans Support Trump Starting Program to Deport All Illegal Immigrants

Trump Nominates Pam Bondi for Attorney General

Netanyahu Cries 'Antisemitism' After International Criminal Court Issues Warrant for His Arrest

Matt Gaetz Withdraws from Consideration as Attorney General
 Ask about published reports of 470 completed suicides of people on antidepressants since Prozac debuted in 1988 and the drug industry will say that's depression for you. Without our drugs, it would be worse. But how does Eli Lilly and Co. explain the mounting suicides of people given Cymbalta (duloxetine) for urinary incontinence or peripheral neuropathy? The planned debut of Cymbalta, now Lilly's number two drug, was even delayed by the suicide of a non-depressed person in 2004. Traci Johnson, a healthy 19-year-old college student volunteer enrolled in a Cymbalta trial hung herself by a scarf from a shower rod in Lilly's Indianapolis, IN laboratory while withdrawing from the drug. Johnson showed no outward signs of depression and Lilly said she had been screened for mental problems, but staff conducting a concurrent Cymbalta trial made comments about her "mental history" to at least four participants--perhaps to keep them from dropping out of the trials as a fifth of volunteers did after the suicide. After Johnson's death, Lilly was ordered to stop accepting new volunteers for the study and to have continuing participants evaluated by an independent psychiatrist and sign new consent forms. But in another Cymbalta trial of 4,124 depressed patients just weeks later, four more participants took their lives according to a Lilly clinical psychiatrist, Dr. John R. Hayes. Meanwhile reports from abroad where duloxetine was already in use as a stress urinary incontinence treatment called Yentreve were just as grave. Twice the expected number of suicide attempts among middle aged women were seen with the drug-- 400 per 100,000 person-years versus a baseline of 160 per 100,000 person-years--said the FDA on its Web site in June, 2005, leading Lilly to precipitously withdraw its application to sell Yentreve in the US. Safety data for Cymbalta and Yentreve obtained by reporter Jeanne Lenzer under a Freedom of Information request for a Independent on Sunday article disclosed 41 deaths and 13 suicides which did not include Johnson's or the four cited by Hayes, says Lenzer. In fact in an article titled," Duloxetine: new drug. For stress urinary incontinence: too much risk, too little benefit," in the December 2005 issue of French medical journal Prescire International, the authors conclude that the drug should not even be in use. More than 40 different types of adverse effects have been reported, including suicide attempts and potentially severe hepatic disorders. (7) Duloxetine is metabolised by the cytochrome P450 isoenzymes CYP 1A2 and CYP 2D6, creating a risk of interactions with other drugs that follow these metabolic pathways. (8) In practice, purely symptomatic treatments that have no documented efficacy but many adverse effects should not be used, especially when there is an alternative treatment with a positive risk-benefit balance. The FDA approved Cymbalta for depression and diabetic peripheral neuropathy in 2004 and generalized anxiety disorder in 2007. But last fall it ordered Lilly to stop downplaying liver toxicity in its promotional materials. And an article in the January 2007 issue of Diabetes Care found Cymbalta actually raises fasting blood glucose which can worsen the diabetic peripheral neuropathy it is supposed to treat. (see: depression; causes and effects) Cymbalta has other side effects too, say users in email messages to a reporter. In Jim Ellsworth, 52 of Houlton, Maine it caused a "hypertensive crisis" that did not fully subside until a month after quitting the drug he says. It caused "involuntary twitching and jerking motions" that persist three years after being off Cymbalta for fibromyalgia says Lonna. And for Amy, who had no history of depression but was "given Cymbalta for Attention Deficit Disorder, (which I haven't been able to find anywhere that it is even approved for that)" it caused a withdrawal in which she "went on rampages and cried over everything and felt so sick and awful that I didn't think I wanted to live anymore." But worst are the out of character, almost matter of fact suicides like a man described by his family as, "NOT depressed, he was a fun-loving guy with all sorts of plans in place for the future," who was prescribed Cymbalta for foot pain. He "had a normal day at work, drove home, said he was going to grab a sandwich to his wife, and went and shot himself." With its number one drug, Zyprexa on the ropes--Alaska and Louisiana sued Lilly for the Medicaid costs of treating Zyprexa-caused diabetes and CEO John C Lechleiter was caught promoting it off label by the New York Times--Lilly is marketing Cymbalta like its pipeline depends on it. It used 500 sales reps from slick contract sales organization Quintiles International to launch Cymbalta as well an unspecified number of its own reps and hopes to get approval for fibromyalgia while doctors are still writing the drug. But bad press for Cymbalta might start sooner than Lilly expects--in a wrongful death suit of Carol Gotbaum who strangled herself with handcuffs while in police custody at the Sky Harbor International Airport in Phoenix, AZ in September. Press reports say the 45-year-old daughter-in-law of a New York City elected official and prominent labor leader was under the influence of Cymbalta. Martha Rosenberg is staff cartoonist on the Evanston Roundtable. She can be reached at [email protected] |



