Microchip Implants Cause TumorsDamning research findings could spell the end of VeriChipAntiChips.com Sep. 10, 2007 |
Popular 
FL State Sen. Randy Fine Celebrated Israel Killing an American - Trump Just Endorsed Him For Congress
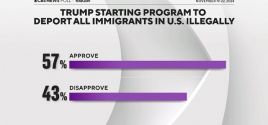
Poll: 57% of Americans Support Trump Starting Program to Deport All Illegal Immigrants

Trump Nominates Pam Bondi for Attorney General

Netanyahu Cries 'Antisemitism' After International Criminal Court Issues Warrant for His Arrest

Matt Gaetz Withdraws from Consideration as Attorney General
 The Associated Press will issue a breaking story this weekend revealing that microchip implants have induced cancer in laboratory animals and dogs, says privacy expert and long-time VeriChip opponent Dr. Katherine Albrecht. As the AP will report, a series of research articles spanning more than a decade found that mice and rats injected with glass-encapsulated RFID transponders developed malignant, fast-growing, lethal cancers in up to 1% to 10% of cases. The tumors originated in the tissue surrounding the microchips and often grew to completely surround the devices, the researchers said. Albrecht first became aware of the microchip-cancer link when she and her "Spychips" co-author, Liz McIntyre, were contacted by a pet owner whose dog had died from a chip-induced tumor. Albrecht then found medical studies showing a causal link between microchip implants and cancer in other animals. Before she brought the research to the AP's attention, the studies had somehow escaped public notice. A four-month AP investigation turned up additional documents, several of which had been published before VeriChip's parent company, Applied Digital Solutions, sought FDA approval to market the implant for humans. The VeriChip received FDA approval in 2004 under the watch of then Health and Human Services Secretary Tommy Thompson who later joined the company's board. Under FDA policy, it would have been VeriChip's responsibility to bring the adverse studies to the FDA's attention, but VeriChip CEO Scott Silverman claims the company was unaware of the research. Albrecht expressed skepticism that a company like VeriChip, whose primary business is microchip implants, would be unaware of relevant studies in the published literature. "For Mr. Silverman not to know about this research would be negligent. If he did know about these studies, he certainly had an incentive to keep them quiet," said Albrecht. "Had the FDA known about the cancer link, they might never have approved his company's product." Since gaining FDA approval, VeriChip has aggressively targeted diabetic and dementia patients, and recently announced that it had chipped 90 Alzheimer's patients and their caregivers in Florida. Employees in the Mexican Attorney General's Office, workers in a U.S. security firm, and club-goers in Europe have also been implanted. Albrecht expressed concern for those who have received a chip implant, urging them to get the devices removed as soon as possible. "These new revelations change everything," she said. "Why would anyone take the risk of having a cancer chip in their arm?" |



