Open Letter to the FDA to Stop Corporations from Lacing Foods, Body Care Products, & Supplements with Dangerous NanoparticlesOrganic Consumers AssociationOct. 12, 2006 |
Popular 
FL State Sen. Randy Fine Celebrated Israel Killing an American - Trump Just Endorsed Him For Congress
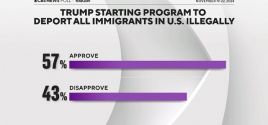
Poll: 57% of Americans Support Trump Starting Program to Deport All Illegal Immigrants

Trump Nominates Pam Bondi for Attorney General

Netanyahu Cries 'Antisemitism' After International Criminal Court Issues Warrant for His Arrest

Matt Gaetz Withdraws from Consideration as Attorney General
 Acting FDA Commissioner Andrew C. Von Eschenbach Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers Lane, Room 1061 Rockville, MD 20852 Dear Commissioner Von Eschenbach, I write to express my serious concerns about the FDA's regulatory oversight of nanomaterials in consumer products. Many consumer products containing engineered nanomaterials are already available on U.S. market shelves, including food and food packaging products. Millions of dollars are being spent by government and industry to apply nanotechnology in areas of food processing, food packaging, and agricultural production. Current nano-food products on the market include a canola oil, a chocolate "slim" shake, a nano-bread, and several nano-food additives and supplements used in soft drinks, lemonades, fruit juices, and margarines. Many food packaging products use nano-composites, nano-clays, and nano-coatings. In addition, if industry observers are correct, hundreds of more new food and agriculture products are under development and many could be on the market in as few as two years. By 2010 the nano-food market will be $20 billion. Many of the world's leading food companies - including H.J. Heinz, Nestle, Hershey, Unilever, and Kraft - are investing heavily in nanotechnology applications. Scientists have found that the fundamental properties of matter can change at the nano-scale, creating physical and chemical properties distinct from those of the same material in bulk form. We know that the new properties of nanomaterials create new risks, like enhanced toxicity. Studies have raised numerous red flags, and many types of nanoparticles have proven to be toxic to human tissue and cells. Nanoparticles can gain assess to the blood stream following ingestion. Once inside the body, the super-tiny size of these materials gives them unprecedented mobility and access to the human body; they can access cells, tissues, and organs that larger particles cannot. The length of time that nanoparticles remain in organs and what dose may cause harmful effects remains unknown. It does not appear that FDA is ready for this wave of nano-food products. I am very concerned about the rapid introduction of these potentially hazardous nanomaterials into our bodies and into our environment without adequate scientific study to ensure that we understand their risks and can prevent harm occurring to people and the environment. The FDA's failure to undertake or review new testing of these nanomaterials despite these known and foreseeable dangers suggests the agency's review process is not acting to ensure consumer health and safety. For these reasons, I strongly request that FDA use its upcoming Public Meeting and its new Nanotechnology Task Force to discuss the human health and environmental risks presented by nanomaterials in consumer products, including food and food packaging products. FDA should act quickly to shore up its regulation of these substances to account for their fundamentally different properties and their associated dangers, including require new nano-specific testing and the labeling of all nanomaterial products, including nano-food products. Currently, FDA's reliance on manufacturers' assurances of safety make me and my family into guinea pigs. FDA must instead independently review all testing and assess the safety of these products as well as force manufacturers to label their nanomaterial products. Only with labeling can I make educated decisions about what I buy and put in and on my body. Until such actions are taken, I fully support a moratorium on the manufacture of nanomaterial consumer products and the recall of products currently on the market. Ronnie Cummins National Director Organic Consumers Association Finland, Minnesota 55603 |



