'Miracle' cancer drug may hurt heartPhiladelphia InquirerJul. 24, 2006 |
Popular 
FL State Sen. Randy Fine Celebrated Israel Killing an American - Trump Just Endorsed Him For Congress
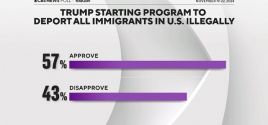
Poll: 57% of Americans Support Trump Starting Program to Deport All Illegal Immigrants

Trump Nominates Pam Bondi for Attorney General

Netanyahu Cries 'Antisemitism' After International Criminal Court Issues Warrant for His Arrest

Matt Gaetz Withdraws from Consideration as Attorney General
 Doctors at Thomas Jefferson University have discovered why the wonder drug Gleevec, heralded for turning off cancer cells, may also cause congestive heart failure in a small number of patients. The implications of the research may also extend beyond Gleevec, revealing potential pitfalls in a whole class of cancer drugs that work in a similar way, the report's authors say. The study follows up on 10 patients who developed severe heart failure after taking Gleevec. Those cases were first reported in 2004 by the University of Texas M.D. Anderson Cancer Center in Houston. But scientists did not know why Gleevec caused the heart to fail. The followup study, published online yesterday in the journal Nature Medicine, details for the first time how Gleevec inadvertently targets a protein maintaining cells that contract the heart muscle and help to force blood through the body. "This finding is a big surprise," said Thomas L. Force, a cardiologist researcher who led the Thomas Jefferson University team and conducted the study with Jean-Bernard Durand of the Texas cancer center. Gleevec is the first of a new class of cancer drugs designed to focus on a single cancer protein and avoid many side effects of previous cancer drugs. Gleevec, also called imatinib mesylate, is used to treat chronic myelogenous leukemia, a bone and blood disease in which the body produces a flood of cancerous white blood cells. It's also used to treat gastrointestinal tumors. Novartis, which makes the drug, said it did not track how many patients take it. About 4,500 or so patients are diagnosed annually with CML. Novartis called the side effect rare, and said that patients who show symptoms are easily treated with standard medications. "The observations of this preclinical study do not change the positive benefit/risk ratio of Gleevec for thousands of patients being treated for cancer and other life-threatening diseases," Novartis wrote in a prepared statement. The company said it had already reported the 10 cases of heart failure to health officials and the side effect is now included on the drug's warning label. Force also predicted the number of heart cases would be small. "The last thing we want to do is cause a Gleevec scare," Force said. "Patients need to be on this drug, so the question is how to keep them on it without it having an effect on the heart." He said patients can be treated for heart failure with treatments such as ACE inhibitors and beta-blockers, but stressed that oncologists and cardiologists would need to work together to monitor patients. Dr. Mitchell Smith, the director of blood disorders at Fox Chase Cancer Center, said the study conveys an important warning to doctors to begin looking for the side-effect. "I have patients with underlying heart disease who have been on Gleevec for years and have not had any problems," Mitchell said. But it's possible oncologists may have missed some cases of congestive heart failure because the symptoms resemble the side effects of cancer treatments, such as shortness of breath and water retention, he said. Before Gleevec's approval, many CML patients died within three to five years. Much of the drug's development began in Philadelphia in the 1960s when Fox Chase and University of Pennsylvania researchers struck upon the first genetic abnormality associated with human cancer. Called the "Philadelphia chromosome," it plays a role in the formation of a gene called BCR-ABL from two broken genes. The fused gene produces an enzyme that allows white blood cells to grow out of control, clogging the body. Novartis targeted this enzyme and found a way to inhibit it with Gleevec. The new drug works more like a sniper than a bomb, hitting the cancer but not other organs, as cancer care often does. The drug was so successful at treating CML that it was approved in 2001 by the FDA in just two months, unusual for a cancer drug. That year, doctors at M.D. Anderson's Cardiomyopathy Center, noticed a patient had developed congestive heart failure. In time, they discovered 10 patients with previously normal heart function who developed heart failure within 2 to 14 months after taking Gleevec. Force and his team soon discovered that the second part of the BCR-ABL gene Gleevec targets - the ABL part - also plays a role in maintaining cells that contract the heart's left ventricle. When Gleevec destroys ABL, heart cells begin to die. "The value here is that we've identified a mechanism, we know about problem and we already have a cure," said Durand. "Now we need to educate physicians." Durand, Force and several major cancer centers hope to track the incidence of heart failure through a novel database tracking system. More study is needed to determine why Gleevec causes the side effect in some patients and not others. He said all 10 patients responded well to ACE inhibitors and the beta-blocker carvedilol. Force and Durand said there are new drugs in the same class as Gleevec, called tyrosine kinase inhibitors, that should also be monitored. More importantly, added Force, doctors should closely monitor new classes of similar drugs that target a wider array of genes. Durand said the work also shows problems with clinical trials. "In cancer trials, if you have a significant cardiac history you're excluded from study, so we take perfect patients," he said. "But doctors don't always prescribe medications to perfect patients and all these problems begin to appear post approval." |



