FDA admits thousands of common medications never received FDA approvalNewsTargetJul. 14, 2006 |
Popular 
FL State Sen. Randy Fine Celebrated Israel Killing an American - Trump Just Endorsed Him For Congress
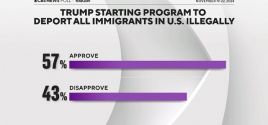
Poll: 57% of Americans Support Trump Starting Program to Deport All Illegal Immigrants

Trump Nominates Pam Bondi for Attorney General

Netanyahu Cries 'Antisemitism' After International Criminal Court Issues Warrant for His Arrest

Matt Gaetz Withdraws from Consideration as Attorney General
 The FDA has admitted that several thousand prescription and over-the-counter medicines -- including cough medicines, painkillers, sedatives and anti-inflammatory drugs -- have never been certified as safe and effective by the regulatory agency. "We consider it a significant and serious drug safety issue that must be addressed since these products may pose a risk to consumers," said Steven Galson, director of the FDA's Center for Drug Evaluation and Research, in a statement. Some doctors and pharmacists may not know they are prescribing unapproved medicine because some of them have been on the market for such a long time. Many of them are listed in the Physicians' Desk Reference and advertised in medical journals. "I am a little surprised there are so many of these prescription medicines out there that haven't been already removed from the market," said John Colaizzi, dean of the Rutgers University Ernest Mario School of Pharmacy. "For me as a pharmacist and for the public, there is an assumption that all the products have been tested and certified for both safety and effectiveness." The FDA admits it does not have complete data on the unapproved medications, but the major categories include some cough and cold medicines with antihistamines, and some single-ingredient narcotics and sedatives. According to FDA spokespeople, most of the unapproved drugs were on the market before the 1962 crackdown on drug laws by Congress, but they say such medicines make up less than 2 percent of the market. Even so, the agency announced it would begin looking into the unapproved prescription medications, paying special attention to those that could present safety risks or lack evidence of efficacy. Perry Cole, president of the Branded Pharmaceutical Association, stands in opposition to this move, saying that nobody knows for sure how many drugs will be affected, and that many have been used for decades to the satisfaction of both doctors and their patients. The FDA says just because a product has been used without any known safety problems does not guarantee its safety or effectiveness. The discovery of so many unapproved drugs currently on the market reveals yet more gaps in the FDA's drug safety approval process, which the agency declares to be the "gold standard" of evidence-based medicine. |



