FDA admits children's antibiotic could cause liver failure, but allows its sale anywayNewsTargetJun. 30, 2006 |
Popular 
FL State Sen. Randy Fine Celebrated Israel Killing an American - Trump Just Endorsed Him For Congress
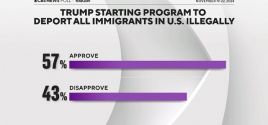
Poll: 57% of Americans Support Trump Starting Program to Deport All Illegal Immigrants

Trump Nominates Pam Bondi for Attorney General

Netanyahu Cries 'Antisemitism' After International Criminal Court Issues Warrant for His Arrest

Russia Advancing in Ukraine at 'Fastest Pace Since Early 2022'
 Though the Sanofi-Aventis antibiotic Ketek has been shown to damage the liver and sometimes cause death after a few doses, the FDA says the drug's benefits outweigh its risks and has allowed the product to remain on the market. Ketek is often prescribed to children. It has been approved to treat sinusitis, bronchitis and mild-to-moderate pneumonia. According to the New York Times, an FDA safety reviewer argued in May that Sanofi-Aventis should stop testing Ketek on children with ear infections. He said that reducing the length of ear pain by one day was not worth risking death. The drug firm has since "paused" its pediatric trials, but according to Dr. John Jenkins of the FDA's Office of New Drugs, discussions are under way to determine if testing can resume. In the United States, 14 adults taking Ketek have suffered liver failure -- four of whom have died -- and 23 others have suffered serious liver injury. In a review of the drug, safety officials determined that while other antibiotics have been shown to damage the liver, Ketek appears to do so four times as often. Dr. Jenkins argues that Ketek should be kept on the market despite its risks because new antibiotics are badly needed. He says antibiotic resistance has rendered many older antibiotics ineffective. However, Senate Finance Committee chairman Charles E. Grassley, R-Iowa, along with Rep. Edward J. Markey, D-Mass., and Rep. Henry A. Waxman, D-Calif., have launched an investigation into the FDA's actions concerning Ketek. "Ketek is another example where the FDA accommodated a drug maker and turned a blind eye to serious safety concerns," Grassley said. |



