Calorie-free stevia's 11-year war with FDANewsdayMay. 02, 2006 |
Popular 
FL State Sen. Randy Fine Celebrated Israel Killing an American - Trump Just Endorsed Him For Congress
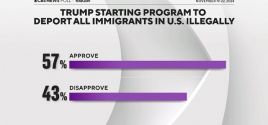
Poll: 57% of Americans Support Trump Starting Program to Deport All Illegal Immigrants

Russia Advancing in Ukraine at 'Fastest Pace Since Early 2022'

Trump Nominates Pam Bondi for Attorney General

Netanyahu Cries 'Antisemitism' After International Criminal Court Issues Warrant for His Arrest
 To many people these days, simply sweetening a cup of coffee is practically akin to picking a poison. Sugar or honey? Too many calories. Equal or Nutrasweet? Too many health risks, especially given recent reports detailing diet soda's dangerously high levels of the cancer-causing compound benzene. So to the sweet-toothed consumer, the increasingly popular, all natural, calorie-free substance called stevia sounds too good to be true. And to the U.S. Food and Drug Administration, it is. For the past 11 years, while artificial sweeteners like Splenda, Equal and Nutrasweet have dominated the diet-conscious market, the stevia industry and the FDA have been at odds over whether the additive poses health risks. But with sales of the plant-based substance, indigenous to South America, growing rapidly in the past few years, stevia's sticky situation is creating an increasingly complex marketplace for consumers, manufacturers and retailers. FDA hard to convince Though the stevia industry promotes it as the only natural, no-calorie way to sweeten foods and drinks and denies any health risks, citing the heavy use of the substance in Japan since the 1970s without any major reported safety concerns, the FDA isn't convinced. Since 1995, the FDA has banned the use of stevia as a sweetener, approving it only for use as a dietary supplement because "available toxicological information on stevia is inadequate to demonstrate its safety as a food additive or to affirm its status as GRAS [generally recognized as safe]." But consumers looking for alternatives to sugar and to chemical sweeteners keep snapping it up. In recent years, the consumption of stevia, which is sold in powder, tablet and liquid form and has a slightly bitter taste, has ballooned. Sales of stevia in the United States reached about $45 million in 2005, up nearly 25 percent from the previous year's sales, according to the Nutrition Business Journal, an industry guide to market research. Once limited to obscure health food stores, stevia can now be found at Trader Joe's stores, Whole Foods and King Kullen stores across Long Island. A well-kept secret But despite its growing consumer base, stevia's long-term prospects are severely limited under FDA regulations. For one thing, most consumers know little to nothing about the plant derivative because government regulations prevent even retail outlets from explaining much about the substance. Even diabetics, sweetener-savvy consumers and potentially some of stevia's most devoted fans, aren't sure what to make of the product. While nutritionists, including those who have worked in conjunction with the American Diabetes Association like Virginia-based consultant Robyn Webb, recommend stevia to diabetics as a safe way to sweeten foods and drinks, the ADA refuses to endorse it because it looks to the FDA for dietary guidelines. And while stevia distributors are pleased with the recent growth of their industry, some worry about the future of their marketplace. "The true growth is in food processors putting it in food products, and that will only come when the FDA approves it," says Warren Sablosky, 52, president of NuNaturals, an Arizona-based stevia distributor that sells pure extract to Wild Oats and Whole Foods Markets. "A lot of big food producers don't want to sit on the legal line." But some have taken the plunge. In January 2004, Steaz, a Pennsylvania-based natural soda manufacturer, introduced a diet line made with stevia rather than aspartame or Nutrasweet. To comply with the legal guidelines, the company can't market it as a soda or even as a beverage (it calls the product a dietary supplement) and must list "supplement facts" rather than "nutrition facts" on its back label. Sales on the rise But for Steaz, the marketing maneuvering was worth the trouble. The company's diet black cherry flavor is now its top-selling item at national grocery chain Wild Oats, and sales of the diet line have increased 200 percent over each of the past two years, according to Eric Schnell, 35, co-founder of Steaz. "The natural community has embraced the brand," Schnell says. Still, the general public, even consumers wary of artificial sweeteners, may not be quite as quick to make the switch. "It's not as good as sugar," said Sigal Elias, 41, of Great Neck, as she tried a tiny taste of pure stevia alongside her two children, Edan, 13, and Romi, 11, at a recent Earth Day celebration outside Grand Central Terminal. "Usually, we drink diet soda, but now we're trying to eliminate it. Because of the side effects, we're kind of concerned," she admitted, "but we love the flavor of Splenda." |



